Dystrophic mdx mouse myoblasts exhibit elevated ATP/UTP-evoked metabotropic purinergic responses and alterations in calcium signalling
Abstract
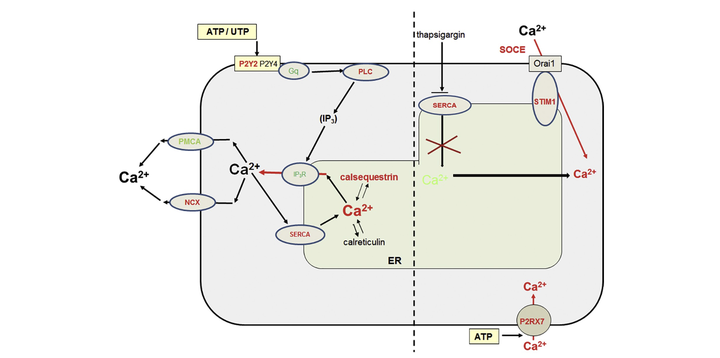
Pathophysiology of Duchenne Muscular Dystrophy (DMD) is still elusive. Although progressive wasting of muscle fibres is a cause of muscle deterioration, there is a growing body of evidence that the triggering effects of DMD mutation are present at the earlier stage of muscle development and affect myogenic cells. Among these abnormalities, elevated activity of P2X7 receptors and increased store-operated calcium entry myoblasts have been identified in mdx mouse. Here, the metabotropic extracellular ATP/UTP-evoked response has been investigated. Sensitivity to antagonist, effect of gene silencing and cellular localization studies linked these elevated purinergic responses to the increased expression of P2Y2 but not P2Y4 receptors. These alterations have physiological implications as shown by reduced motility of mdx myoblasts upon treatment with P2Y2 agonist. However, the ultimate increase in intracellular calcium in dystrophic cells reflected complex alterations of calcium homeostasis identified in the RNA seq data and with significant modulation confirmed at the protein level, including a decrease of Gq11 subunit α, plasma membrane calcium ATP-ase, inositol-2,4,5-trisphosphate-receptor proteins and elevation of phospholipase Cβ, sarco-endoplamatic reticulum calcium ATP-ase and sodium‑calcium exchanger. In conclusion, whereas specificity of dystrophic myoblast excitation by extracellular nucleotides is determined by particular receptor overexpression, the intensity of such altered response depends on relative activities of downstream calcium regulators that are also affected by Dmd mutations. Furthermore, these phenotypic effects of DMD emerge as early as in undifferentiated muscle. Therefore, the pathogenesis of DMD and the relevance of current therapeutic approaches may need re-evaluation.